The new article (original in German) explains the energy transition from a simple but fundamental physical perspective. And something very basic sooner or later leads us to entropy. It helps us understand why electricity-based technologies are much more efficient than those that use combustion and heat as an intermediate step. And why the energy transition is therefore much more of a technical revolution, namely away from heat and towards electricity, with significantly higher efficiency.
What does the energy transition have to do with entropy? Entropy is a physical quantity that determines how much energy we can use. So we need entropy for efficiency considerations.
More precisely, what we need are differences in entropy. These occur when there is a difference in temperature. This difference can then be used to generate usable energy. Every thermal power plant works in this way when it generates usable energy in the form of electricity. The greater the temperature difference, the more usable energy can be obtained.
How hot can it get during combustion?
This leads us to the fundamental question of what maximum temperature we can actually achieve during combustion. This is nothing new, of course; the concept is called adiabatic flame temperature. It can be estimated quite simply: combustion generates heat, so we achieve the highest temperature when the heat released warms the least amount of air. With conventional fuels, this leads to temperatures of around 2500-3000 degrees centigrade.
These temperatures are not usually reached, as metals would melt. But if only a lower temperature is used, we are already wasting some of the usable entropy. And if we burn fuel just to keep our living room at 20 degrees centigrade, we are wasting most of the usable energy.

If we then take into account that photosynthesis produces fuels with very low efficiency, typically with an efficiency of 1%, then the whole process is simply very, very inefficient. You can read more about why photosynthesis is so inefficient in this blog post.
How can we do better? With electricity!
The answer is simple: avoid combustion and, more generally, heat as an intermediate step. Electricity directly from photovoltaics, not from combustion. Mobility from electricity, not from combustion. Room heating via heat pumps, not from combustion. This is much more efficient, as we then need less energy to achieve the same goal.
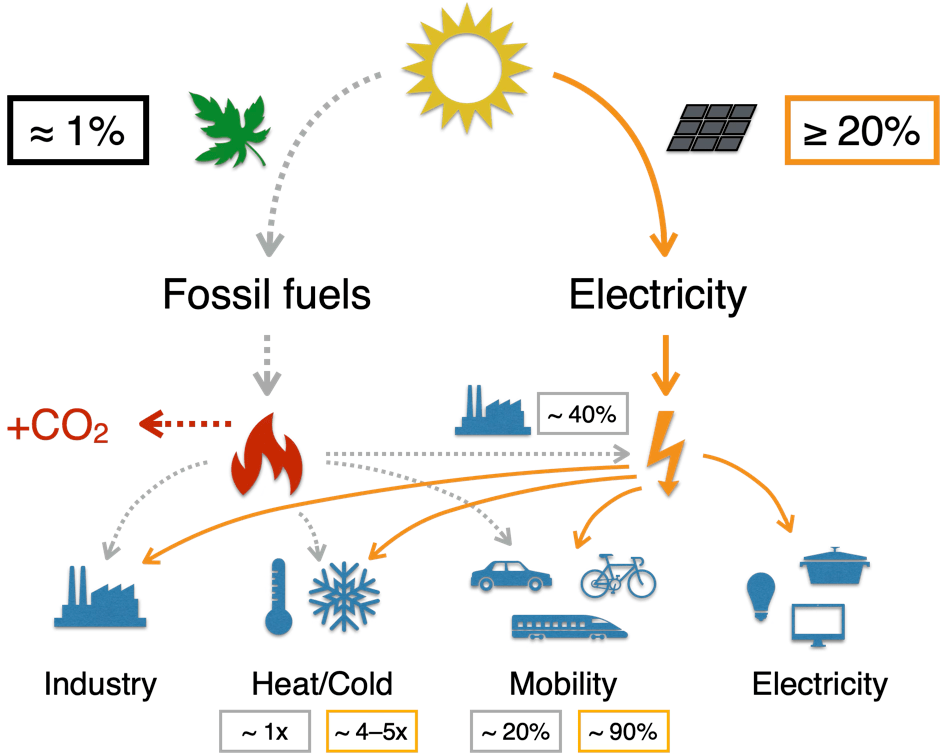
In doing so, we are following a technological development that we have long since achieved elsewhere. Light is nowadays generated directly from electricity using LEDs, rather than through combustion or hot filaments. Trains run mainly on electricity; steam locomotives no longer exist. We are seeing the same trend in electricity generation in Germany – lower use of primary energy while maintaining the same level of electricity generation. This is because PV and wind do not burn anything. For good reason, electricity-based technologies are simply superior. This allows primary energy consumption to be reduced significantly. It can then be more easily covered by PV, wind and storage. You can find out more about this in the article (translated in English on arXiv).
What I find wonderful about this article
At the end, I would like to add a brief personal comment. On the one hand, I am very happy and satisfied with this article because it describes the energy transition in a very simple and fundamentally physical way as a huge leap in efficiency. And it puts entropy in a really practical and relevant context. I believe that this focus on the essentials is ultimately the core of science.
On the other hand, I find this insight really important because it gives the whole discussion about climate change and the necessity of the energy transition a completely different, more fundamental justification. The energy transition is not a green-tinged ideology, it is not a luxury. It is a completely natural trend, it follows from physics. And if we miss the boat, we will be left behind technologically. The faster we implement it, the more we will be involved in the necessary innovations and remain economically successful.
So now it is ‘only’ a matter of resolutely countering the overwhelming influence of the enemies of freedom and progress and doing the right thing.
Reference
Axel Kleidon and Harald Lesch (2025) Electricity instead of heat: Avoiding combustion significantly reduces primary energy demand. Physik in unserer Zeit, published online/in print. https://doi.org/10.1002/piuz.202501742. English translation on arXiv: https://arxiv.org/pdf/2506.12714


This article provides an excellent explanation of why electricity-based technologies are far more efficient than combustion-based ones. The connection to entropy makes the energy transition much clearer—it’s not just “green ideology,” it’s physics driving a more efficient and sustainable future. Thanks for highlighting how adopting electricity directly, through PV, wind, and heat pumps, can dramatically reduce primary energy demand. check bill here: https://efescobill.pk
LikeLike